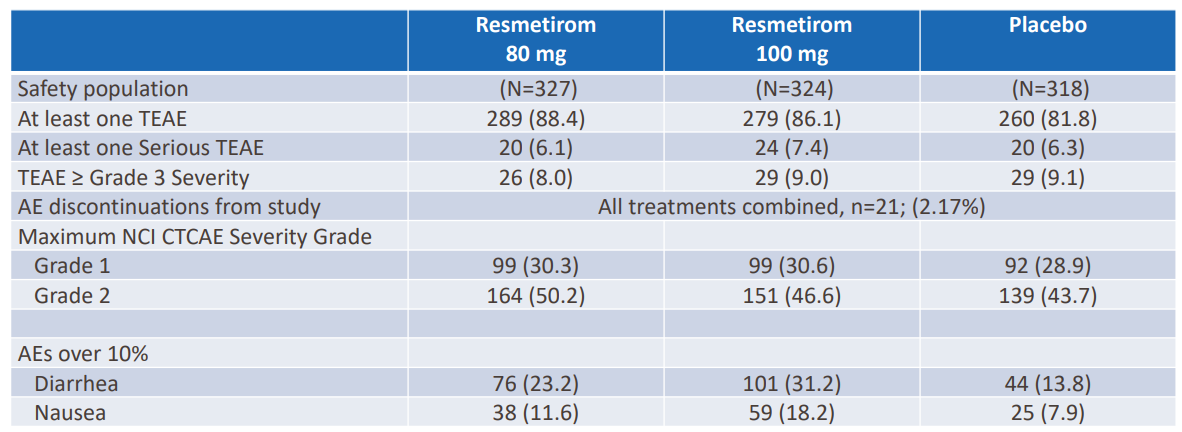
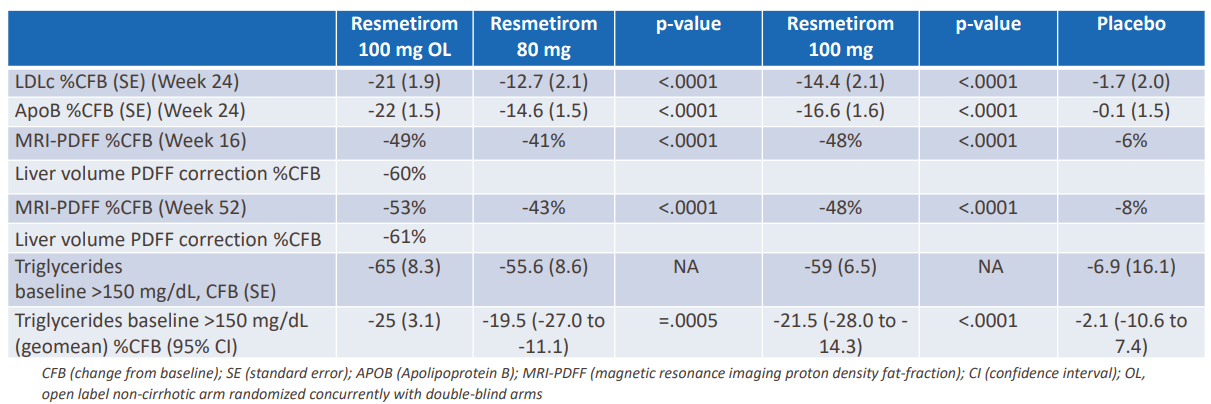
HIGH IMPACT – At its 4Q/full-year 2021 earnings release, Voyager announced a license agreement with Novartis for the TRACER AAV capsids.
- Under the agreement, Novartis may exercise options to license novel AAV capsids generated from Voyager’s TRACER capsid discovery platform for potential use with three CNS targets and options to access capsids for two additional targets in the future. These targets are distinct from those being explored in Voyager’s internal pipeline and other licensing agreements.
- Voyager will receive $54 million up front and is entitled to receive up to $27.5 million in exercise fees for three initial CNS targets, exercisable by Novartis within 12 months of signing.
- Novartis may elect to evaluate capsids for up to two additional targets in the future for $18 million upon selection of each target, and a $12.5 million exercise fee for selection of a capsid for each target.
- Voyager is also eligible to earn up to $1.5 billion in total development, regulatory, and commercial milestones for products utilizing Voyager-licensed capsids, as well as mid- to high-single-digit tiered royalties based on net sales of Novartis products incorporating the licensed capsids.
Voyager also reiterated the license agreement with Pfizer in October 2021 that allowed Pfizer to receive target-specific access to Voyager’s TRACER capsids for one CNS and one cardiovascular target.
Preclinical data, previously presented at ASGCT 2021, demonstrated AAV9-derived TRACER capsids can be delivered across blood-brain barrier when dosed intravenously.
The company plans to present preclinical data on additional TRACER AAV9 and AAV5 variants aimed to improve CNS targeting at a spring medical conference (likely ASGCT 2022).
Additional pipeline assets include gene therapy programs Parkinson’s, ALS, Huntington’s, and Alzheimer’s. Preclinical data will be presented at a spring medical conference.
Q&A:
- On Pfizer partnership progress to-date: There is a 12-month evaluation period during which we provide the construct necessary for Pfizer to evaluate the capsids, so that’s underway. It’s in process. They let us know which capsids they’d like to evaluate right now, and that work is ongoing. So we’ll find out whether in which capsids they like to exercise on. They have until October of this year to make that decision.
- On Novartis program’s CNS indications: the 3 CNS targets under the Novartis deal are undisclosed. So we can’t comment on what those are, except that they are in the CNS. So there is a focus on rare diseases in CNS just in terms of the limitations of the agreement that will be disclosed in due course, but it’s not really very limiting when we talk about what rare disease is with respect to CNS.
- On Voyager’s advancement of internal pipeline moving towards clinic: With regard to the pipeline, it’s an early stage pipeline. We rebooted last August and really began to focus the pipeline entirely on the tracer capsids. So this means intravenous delivery for a number of the targets, including the CNS targets that we have in the pipeline. We have not released any time lines at this point. But suffice to say, it’s early stage. We are developing the capsids. We’re developing the production processes and doing the requisite preclinical work to move those into the clinic.
- On business development strategies: Plans to take advantage of the TRACER platform and focus on getting out the capsids to some other major players in the field to allow those programs to move forward. We have made a big shift in our pipeline and there’s work to be done there. We’ll continue to move forward with our own proprietary pipeline and we’ll continue to do transactions. Will consider targets within CNS and beyond CNS. Will not prosecute every target in CNS. Will also look for opportunities in traditional collaborations where we work on programs together with partners. Also, these early transactions are also helpful to strengthen balance sheets and allow us to make investments and move the company forward.
- On regulatory hurdles of moving TRACER programs to clinic: There are no particular specific gating factors other than the normal gating factors required for putting in AAV gene therapy product into the clinic, which does have to follow a specific pathway based upon safety and efficacy. With regard to the novel capsids, those functions like the conventional AAVs. They just have different tropism and will provide a safety profile that we are anticipating based upon the preclinical work that we’ve done. It should be substantially different and the safety profile that’s being offered by the conventional AAVs. And so what that means is that while we will need to be on high alert for any safety issues as everyone is with the conventional AAVs, we’re anticipating a much broader therapeutic window with these capsids because they’ve been designed to offer that.
- On updates of VY-AADC program in PD after DSMB assessment: We haven’t provided any updates with regard to the discussions or the progress on the program. The program was put on hold some time ago, and where that remain in that position that there are significant challenges in terms of the technical aspects of that. So we don’t expect to be providing significant updates on that program at this time. The clinical work that we did with Parkinson’s as well as the preclinical work with Huntington’s disease, both involved intraparenchymal delivery of AAV. We are of the opinion at this point that that is not necessarily a good idea. That is the reason why the TRACER platform offers us so much potential, the ability to dose intravenously get across the blood brain barrier in sufficient quantities to offer a therapeutic effect. So we have switched our entire path pipeline and planning over to intravenous delivery of these capsids for CNS diseases as well as for non-CNS diseases
- On Novartis’ selection of capsids, 3 capsids vs 1 capsid for the three targets in the deal: Could be 3 capsids for 3 targets, or 1 capsid that does all. Some of our best capsids maybe workhorses that can be applicable for multiple targets. In fact, it may be the case that Voyager uses 1 capsid for 1 or more of its own pipeline programs and 1 or more of our partners might end up using the same capsid for some fairs.
- On safety concerns of AAV gene therapy such as liver toxicity and complement activation: TRACER capsids are offering 2 possibilities to increase the therapeutic window. The first possibility is that we can simply use a lower dose of the capsid, substantially lower dose. And by doing that, we are providing a lower dose to the organs and tissues that are subject to the toxicity. The second aspect of this is that we have noticed in some of our TRACER capsids that they are detargeting specific organs. We think that expanding that therapeutic window in both directions will offer us some safety advantages over the conventional capsids that don’t have much of the therapeutic window.
inThought Analysis
Agreements with Novartis and Pfizer validate the potential of Voyager’s TRACER capsid platform. The new transaction agreement also extends Voyager’s cash runway.
Voyager has previously shifted priorities in its pipeline to fully invest in the TRACER capsid platform. The company continues to differentiate the TRACER capsids from traditional AAV gene therapy. Voyager boasts the platform can enable the development of gene therapies that can target desired cells and tissues with high specificity at low doses and avoid off-target risks. It remains to be seen whether the new capsids can demonstrate better safety profile that addresses many of regulatory concerns for AAV gene therapies. Regardless, vector capsid technologies and delivery techniques will be the key to future generations of gene therapy.
Source: Voyager 4Q/FY 2021 Earnings Release, Voyager 4Q/FY 2021 Earnings Webcast