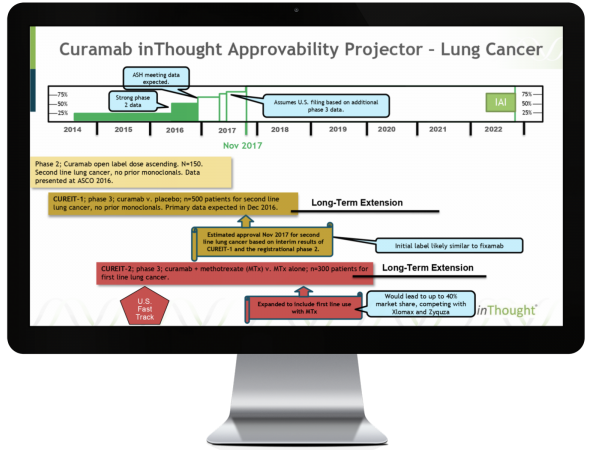
The inThought Approvability Index (IAI) is a dynamic tool that assesses the progress of a drug candidate through clinical development. This tool can be used to evaluate strength of clinical data and trial design, benchmarked against historical parameters and the drug’s likelihood to maintain forward momentum.
inThought analysts scrutinize all aspects of development (preclinical to current phase), validate hypotheses with key opinion leaders and investigators, and assign points for specific line items relating to safety, efficacy, tolerability and other factors.
Historical Approval Rates
Possible points total 100 upon drug approval and are allocated in each phase according to the historical approval rate of similar drugs, such that the current points of a drug relate to its probability of approval.
Current Approval Track
A letter grade is assigned and reflects the momentum of a drug candidate in its current phase, with “A” indicating significantly above average/likely to progress, “C” indicating average, and “F” indicating significantly below average/unlikely to progress.

inThought Research empowers biopharma leaders with comprehensive strategic insights. We combine Wall Street financial acumen and swiftness, scientific expertise, and advanced AI analytics to navigate the complex landscape of drug development.
© 2025 inThought Research. All Rights Reserved.


As the president of inThought Labs, Chris is focused on constantly improving inVision, the leading competitive and market intelligence platform for the biopharmaceutical industry, to better meet the changing needs of clients.
With 20 years of experience in roles being a consumer of market and competitive information, Chris understands the needs and priorities of clients. Chris was a senior principal and co-founder of inThought, a life science consulting, market research, and analytics firm. Collaborating with Ben Weintraub, Chris also co-founded BiotechTracker, an online tool for investors and precursor to inVision. Previous to inThought, he was a healthcare analyst and co-portfolio manager at two investment firms. Chris served in health care policy roles at the White House Office of Management and Budget. These roles included Medicare Desk Officer at the Office of Information and Regulatory Affairs, where he was responsible for providing recommendations to senior White House policy officials on healthcare policies and regulations.
Chris has a Master in Business Administration from Harvard Business School, a Master in Engineering from Villanova University, and a Bachelor of Science in Engineering from Cornell University. Prior to attending Harvard Business School, Chris served on two U.S. Navy nuclear submarines and at the Pentagon.