HIGH IMPACT – Madrigal announced positive topline results from the pivotal Phase 3 MAESTRO-NASH trial of resmetirom (THR-β agonist) in non-cirrhotic NASH with liver fibrosis. MAESTRO-NASH achieved both liver histological endpoints and potentially clinically meaningful effects with 80 mg and 100 mg doses of the drug, relative to placebo.
Study summary:
- 52-week phase 3 study in F1B-F3 patients
- >1,000 patients enrolled in US and Europe for wk 52 analysis
- Patients randomized 1:1:1 to receive resmetirom 80 mg, resmetirom 100 mg, or placebo taken orally once daily
Efficacy results:
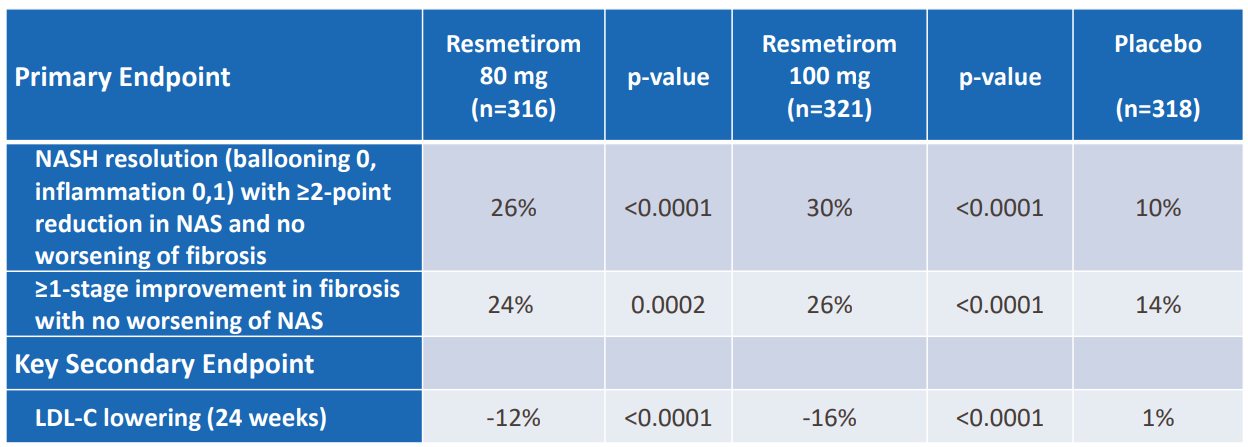
- Dual primary endpoints of NASH resolution and no worsening of fibrosis and ≥ 1-stage improvement in fibrosis with no worsening of NAS were met
- Key secondary endpoint of LDL-C lowering was also met
- Biopsies were read by two central pathologists; each pathologist’s score showed statistically significant magnitude of response at both doses for both histology endpoints
- Endpoints were achieved independent of baseline fibrosis stage and diabetes status
- Additional secondary histology endpoints were achieved at both doses; ≥2 point reduction in NAS with no worsening of fibrosis, ≥2 point reduction in NAS with ≥1-stage improvement in fibrosis, NASH resolution (with ≥2 point reduction in NAS) with ≥1-stage improvement in fibrosis and 2-stage reduction in fibrosis without worsening of NAS
- Statistically significant reduction from baseline in liver enzymes, reductions in atherogenic lipids and lipoproteins, fibrosis biomarkers and imaging tests (MRI-PDFF, CAP and liver stiffness measures) was reported
Safety results:
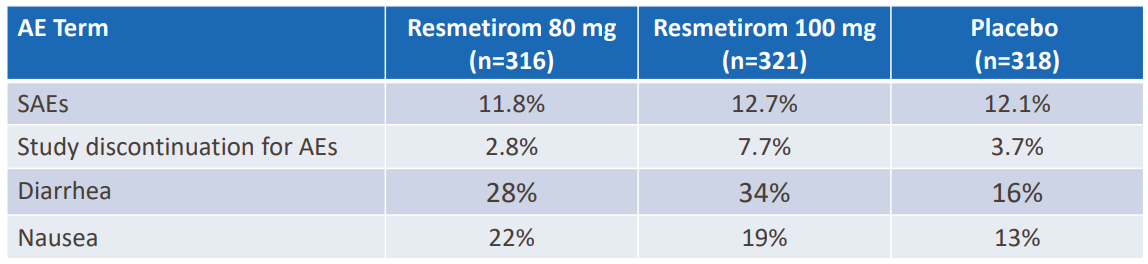
- Resmetirom was safe and well-tolerated at both the 80 mg and 100 mg doses
- The frequency of SAEs was similar across treatment arms, and the rate of study discontinuation for AEs was low
Upcoming milestones:
- Madrigal intends to file a NDA seeking accelerated approval of resmetirom for the treatment of non-cirrhotic NASH with liver fibrosis in 1H 2023
- The company intends to submit primary results for publication in a peer-reviewed journal and present study results at a future scientific congress
inThought Analysis
- The MAESTRO-NASH results have beaten all expectations in meeting not only both primary endpoints of NASH resolution and improvement in fibrosis but also key secondary endpoints including multiple non-invasive tests. Street reacted positively with Madrigal’s stock tripling following the announcement.
- While Intercept is poised to file for Ocaliva by end of 2022, making it the first of the two to potentially enter the market, the drug has been marred with safety and tolerability concerns, particularly around hepatic safety. Moreover, the phase 3 study in compensated cirrhosis has failed to show any benefit of the drug. In contrast, resmetirom has been shown to be relatively well tolerated, besides the GI issues, and is expected to have a relatively smoother path to approval.
- Both resmetirom and Ocaliva are oral drugs, and therefore have an advantage over other promising late-stage drugs such as semaglutide or efruxifermin, which have to be administered subcutaneously.
- While competitors such as Inventiva’s lanifibranor and Akero’s efruxifermin have achieved both endpoints of NASH resolution with no worsening of fibrosis AND improvement in fibrosis with no worsening of NASH in their phase 2 programs, they are yet to replicate those results in their larger phase 3 programs.
- NASH represents an enormous unmet need, and has historically been a drug development graveyard; a first entrant to the market will enjoy a significant advantage.
- While Intercept already has had significant commercial experience with Ocaliva, which is marketed for primary biliary cirrhosis, Madrigal will have to build up its commercial presence from scratch in order to market resmetirom. These favorable results brings Madrigal to the forefront as a lucrative acquisition target by large pharmas, perhaps Pfizer, Novartis or Amgen.



